De, S., Stadlmayr, G., Rebnegger, C., Mattanovich, D., & Gasser, B.
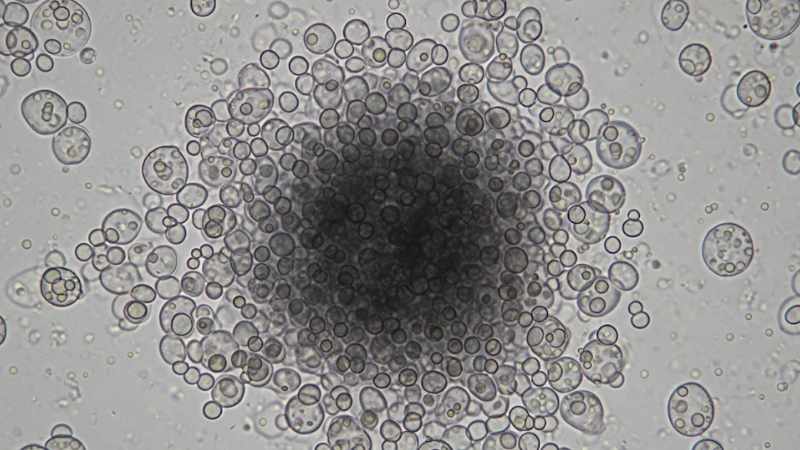
The biotechnologically important yeast Komagataella phaffii exhibits a reversible, pH-dependent flocculation phenotype, which is most pronounced at an acidic pH of 4.0. This study investigates the underlying genetic mechanisms, revealing a complex regulatory network distinct from the model yeast Saccharomyces cerevisiae. Transcriptional analysis identified six flocculin (FLO) genes as differentially expressed at low pH. The transcriptional repressor Nrg1 was identified as a key negative regulator of flocculation, as its overexpression prevents the phenotype. Conversely, Flo8, a master regulator in S. cerevisiae, appears uninvolved. The most striking finding concerns the flocculin gene Flo5-1; its deletion paradoxically results in enhanced flocculation, suggesting it may act as a flocculation antagonist or a sensor. These results highlight the unique regulatory strategies in non-conventional yeasts and provide valuable targets for strain engineering to either prevent or exploit flocculation in industrial bioprocesses.
The non-conventional yeast Komagataella phaffii (formerly Pichia pastoris) is a cornerstone of modern biotechnology, prized for its efficacy in recombinant protein production. Industrial processes typically maintain neutral or slightly acidic conditions, but the yeast’s response to more extreme pH values is less understood. In a recent paper in Applied Microbiology and Biotechnology, a team of researchers led by Sonakshi De and Brigitte Gasser from Austrian research institutions investigates a distinct phenotype: K. phaffii exhibits strong, reversible flocculation when cultivated at an acidic pH of 4.0. This study provides a detailed molecular dissection of this phenomenon, revealing regulatory mechanisms that diverge significantly from those in the model yeast S. cerevisiae.
Methodology and Key findings
The study’s most significant contributions lie in the functional characterization of key regulatory components through gene knockout and overexpression experiments:
- Nrg1 as a Negative Regulator: The transcriptional repressor Nrg1 was identified as a crucial negative regulator of pH-dependent flocculation. Strains overexpressing NRG1 showed a stark reduction in cell aggregation. This positions Nrg1 as a key factor in suppressing this stress response.
- The Paradoxical Role of Flo5-1: In a counter-intuitive finding, the deletion of the flocculin gene flo5-1 resulted in enhanced and faster flocculation. This suggests that, under these conditions, Flo5-1 does not act as a typical adhesin but may function as a flocculation antagonist or a cell-surface sensor. This observation aligns with the qRT-PCR data, which showed that FLO5-1 expression is downregulated at the flocculation-inducing pH of 4.0.
- Divergence from S. cerevisiae Model: In stark contrast to the baker’s yeast regulatory paradigm, neither the master activator Flo8 nor the prominent flocculin Flo11 were found to be essential for the flocculation phenotype in K. phaffii, highlighting the importance of studying these phenomena directly in non-conventional yeasts.
Significance and Avenues for Valorization
For future applications, the engineered `NRG1-OE` strain represents a valuable platform for bioprocesses where flocculation must be avoided. Conversely, understanding how to disable inhibitors like Flo5-1 could pave the way for developing strains with switchable, on-demand flocculation characteristics, thereby optimizing downstream processing. This study opens new avenues for both fundamental research and targeted industrial strain engineering.